What Are the Regulations Around Animal Testing for Drug Development?
Modern drug safety testing on animals didn’t begin in earnest until the 20th century. Laws like the Food, Drug, and Cosmetic Act of 1938 led to a dramatic increase in animal testing during medication development.
While the ethical considerations surrounding its use are often debated, animal testing remains a regulated and essential part of the drug development process to ensure the safety and efficacy of new drugs before they reach human trials.
The Role of Animal Testing in Drug Development
Animal testing is crucial in the early stages of drug development to assess how a new drug interacts with a living organism. This stage is often referred to as preclinical testing and is a necessary step before human clinical trials can begin.
Regulatory agencies such as the Food and Drug Administration (FDA) in the U.S. and the European Medicines Agency (EMA) in Europe require that any new medication undergoes rigorous preclinical testing, which often includes animal testing.
This testing provides valuable information about:
● Drug Safety: To identify any potential toxic effects or adverse reactions that could be dangerous to humans.
● Efficacy: To determine whether the drug effectively treats the targeted condition or disease.
● Dosage: To establish safe dosage levels that will inform human trials.
Regulations Governing Animal Testing
Animal testing is governed by a range of national and international laws, guidelines and ethical codes to protect animal welfare. In most countries, drug developers must comply with strict regulations to ensure that testing on animals is done humanely.
U.S. Regulations
In the United States, The Animal Welfare Act (AWA) is the primary law regulating the treatment of animals in research. Enforced by the United States Department of Agriculture (USDA), the AWA ensures that animals used in research receive proper care and are not subjected to unnecessary suffering.
However, it’s important to note that the AWA does not cover all animals, such as rats, mice and birds, which are commonly used in drug testing. Despite the lack of strict formal regulations, ethical standards and guidelines from institutions like the National Institutes of Health (NIH) and the FDA still set standards for the treatment of all test animals.
European Regulations
In Europe, animal testing is governed by Directive 2010/63/EU, which sets standards for the protection of animals used in scientific research.
This directive emphasizes the Three Rs principle:
- Replacement: Encouraging the use of alternative methods to animal testing wherever possible.
- Reduction: Minimizing the number of animals used in research by improving study design.
- Refinement: Improving procedures to minimize pain and distress in animals.
This directive applies to all vertebrate animals, including fish and amphibians, used in research, and provides strict guidelines on when and how animals can be used.
International Guidelines
On an international level, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has established guidelines for drug development. These guidelines, followed by regulatory authorities around the world, emphasize that animal testing should be conducted only when necessary and when no valid alternative methods are available.
Which Species Are Commonly Used and Why?
Different types of animals are used in drug testing depending on the nature of the drug and the information needed about its effects. The choice of animal is typically guided by similarities between the animal’s biology and human biology, as well as practical considerations related to the specific study.
Commonly Used Animals
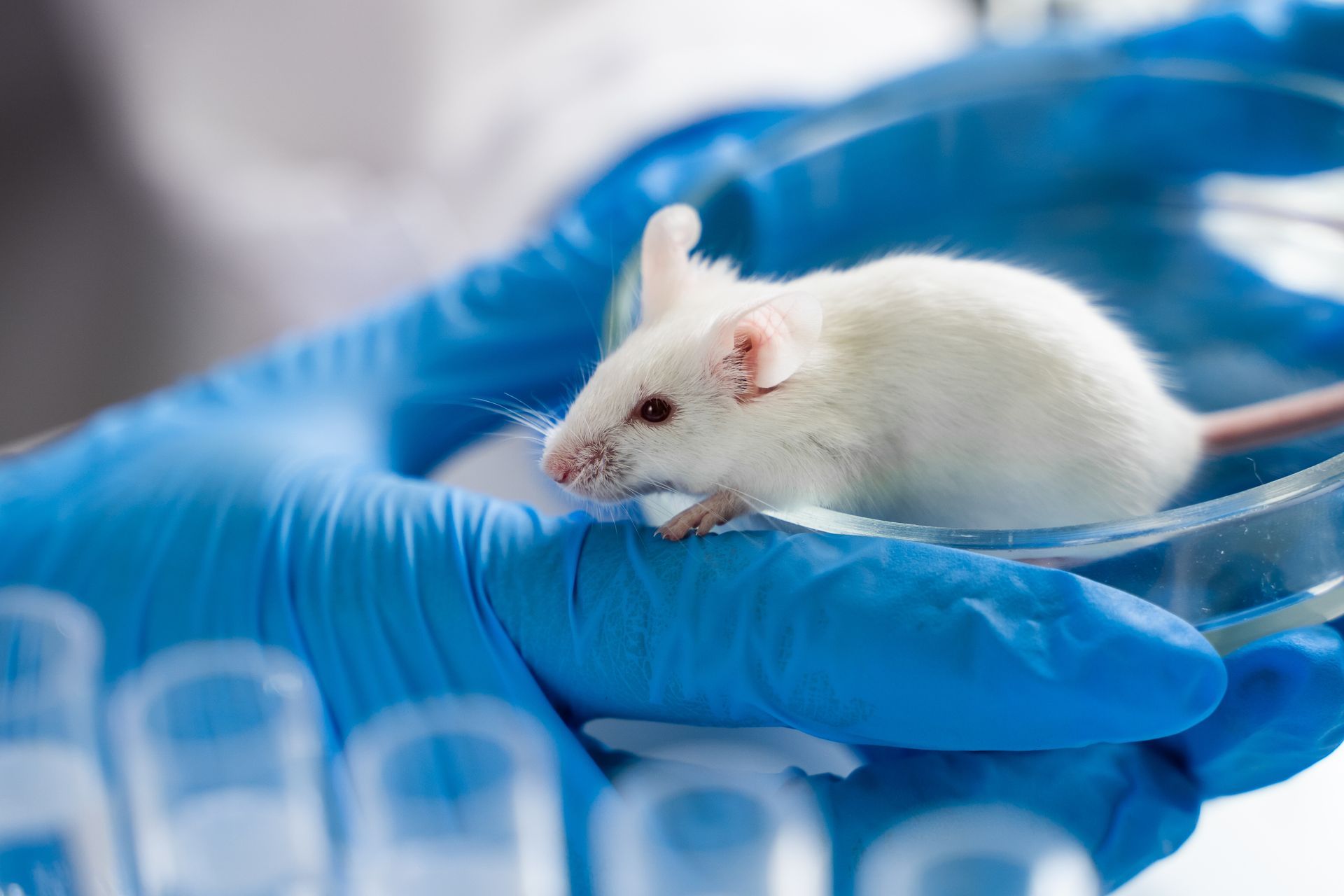
- Rodents (Mice and Rats):
These are the most commonly used animals in drug testing because they have short life cycles, are inexpensive to maintain and have biological systems similar to humans. Their genetic and physiological similarities to humans make them useful for studying how drugs are metabolized and for testing drug safety.
- Rabbits: Rabbits are often used to test for skin irritation or eye irritation caused by drugs or chemicals because of their sensitive skin and eyes. They are also used for testing certain vaccines.
- Dogs:
Dogs, particularly beagles, are sometimes used in testing drugs for conditions like heart disease, as their cardiovascular systems are similar to humans. However, the use of dogs in testing is controversial and is generally reserved for cases where no other suitable alternatives exist.
- Non-Human Primates:
Primates are used in limited cases because of their close genetic relationship to humans, particularly in the development of vaccines or drugs for complex diseases like HIV/AIDS. However, due to ethical concerns, their use is strictly regulated and often a last resort.
- Fish and Zebrafish: In some cases, fish like zebrafish are used for genetic and developmental research. Their transparent embryos and fast reproductive cycles make them useful for studying drug effects on embryonic development.
Alternatives to Animal Testing
In recent years, there has been a growing push toward reducing the use of animals in drug testing. Advances in in vitro testing, computer models and organs-on-chips are helping scientists simulate how drugs will affect human cells and tissues without the need for animal models.
However, these alternatives are not yet fully able to replace animal testing, especially when it comes to understanding how a drug will interact with complex biological systems.
Stay Informed About the Medications You Take in Houston, TX
If you have questions about your prescriptions, our knowledgeable team at St. Hope Pharmacy is here to help. Contact us today to speak with one of our pharmacists and get the information you need to confidently make decisions about your medications and medical devices.


